Paxlovid
Since Paxlovid must be taken within five days after symptoms begin authorizing state-licensed pharmacists to prescribe Paxlovid could expand access to timely treatment for some patients who. 1For information on medical conditions and factors associated with increased risk for progression to severe COVID-19.
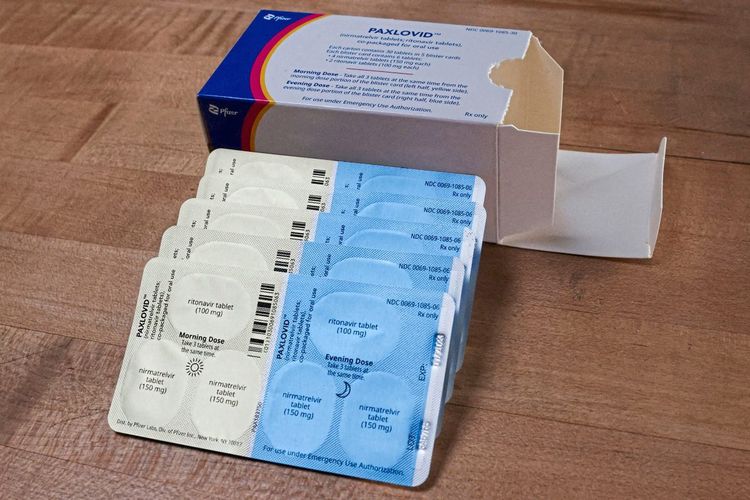
Corona Nicht Nur Joe Biden Hat Einen Paxlovid Rebound Doch Fachleute Beruhigen Gesundheit Derstandard De Wissen Und Gesellschaft
Paxlovid is also contraindicated with drugs that conversely strongly induce those same enzymes leading to the faster breakdown of nirmatrelvir or ritonavir as reduced concentrations of.

. Learn how FDA prepares for and responds to emergencies and how FDA plays a role in protecting the United States from chemical biological radiological nuclear and emerging infectious disease. Since the trigger of long COVID is acute infection with SARS-CoV-2 it makes intuitive sense. Past studies have shown that Paxlovid reduces the risks of hospitalization and death from COVID-19.
National Center for Biotechnology Information. Call 1-800-232-0233 TTY 1-888-720-7489 to get help in English Spanish and more than 150 other languages8 AM to midnight ET 7 days a week. Global information PAXLOVID nirmatrelvir tablets.
Improving patient care through guidelines evidence summaries and decision aids that we can all trust use and share. Paxlovid TM is intended to reduce the severity of COVID-19 symptoms in people at risk of developing serious complications from this infection. The entire pandemic response was completely unnecessary.
Code Prescriber Medicinal Product Pack Name form strength and pack size Max qty packs. Studies from the drugmaker showed that in unvaccinated people at risk of serious COVID. What Prescribers and Pharmacists Need to Know.
Not on the ARTG but approved for import and supply in Australia until 28022023 due to a shortage of another medicine or for public health reasons. Paxlovid effectiveness needs to be assessed in a noncontrolled setting. It is estimated to reduce the risk of hospitalization or death from COVID-19.
Paxlovid the Covid-19 treatment made by Pfizer reduced hospitalizations and deaths in older patients during the Omicron surge in Israel earlier this year but made no difference for patients. Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group on behalf of the Ontario COVID-19 Science Advisory Table. 6 2022 Read the full story here.
This treatment is still being ignored by every mainstream medical institution. Ontario is considering allowing pharmacists to prescribe the COVID-19 treatment drug Paxlovid in order to expand access the provinces top doctor says. This is much better than any COVID vaccine performs in practice and its much safer too.
This document focuses on access to Paxlovid. In this study we used population-based real world data to evaluate the effectiveness of Paxlovid. Government researchers reported on.
Paxlovid was granted emergency use authorization for the treatment of mild to moderate COVID-19 based on the interim analysis of EPIC-HR trial. PAXLOVID is not an appropriate therapeutic option based on the authorized Fact Sheet for Healthcare Providers or due to potential drug interactions for which recommended monitoring would not be feasible. Whether you are a patient caregiver or healthcare professional it is important to report adverse events for Pfizer products.
Also known as PAXLOVID nirmatrelvir tablets. Due to the potential for severe drug-drug interactions with the ritonavir component of Paxlovid it is strongly suggested that healthcare providers not experienced in prescribing this drug refer to. National Center for Biotechnology Information.
Call the Centers for Disease Control and Prevention CDC COVID-19 hotline which can provide information on the Test to Treat initiative. Ritonavir tablets Adverse Event Report. It comes in tablet form.
Health Canada has authorized the use of Paxlovid TM in Canada. But unlike Paxlovid which prevents COVID from replicating in an infected persons body NMT5 alters the virus causing it to gain warheads that temporarily alter the cells where COVID. This is much better than Paxlovid only a 25X reduction if you believe the trial data.
A rebound of COVID-19 symptoms in some patients after taking Pfizers antiviral Paxlovid may be related to a robust immune response rather than a weak one US. Ritonavir The regulatory status of PAXLOVID varies worldwide. Paxlovid eligibility refer to FDAs Fact Sheet for Healthcare Providers.
This is the first oral treatment specifically for COVID-19. We would like to show you a description here but the site wont allow us. Paxlovid a five-day course of pills from Pfizer is at the top of the list of recommended treatments.
Remdesivir an intravenous antiviral medication administered as a three-day course may also be available for people at higher risk of serious illness due to COVID-19 who cannot take Paxlovid or as an alternative to Paxlovid based on clinical assessment. Ritonavir tablets copackaged for oral use also known as PAXLOVID PF-07321332.

Covid Medikament Paxlovid Corona Pille In Deutschland Ladenhuter Tagesschau De

Covid 19 Treatment In 2022 An Faq Carolina Public Press

How To Get Paxlovid A Pill That Helps Prevent Covid Hospitalization Shots Health News Npr

Paxlovid Reduziert Bei Hochrisikopatienten Todesfalle Zm Online
Drohender Verfall Paxlovid Verlangerung Der Haltbarkeit Wird Gepruft

Paxlovid Warum Die Tablette Gegen Corona Kaum Genutzt Wird Zeit Online

Paxlovid Was Muss Beachtet Werden Apotheke Adhoc
Stellungnahme Zur Anwendung Von Paxlovid Und Lagevrio Bei Patienten Mit Ckd Und Bei Nierentransplantierten Dgfn
Paxlovid Scheint Gegen Omikron Zu Wirken Gelbe Liste

Intensivmediziner Fur Vermehrte Behandlung Von Coronapatienten Mit
Pfizer To Deliver Up To Six Million Courses Of Paxlovid To Global Fund
Paxlovid Schon Bei Leichtem Corona Ptaheute
Paxlovid As A Covid 19 Treatment What You Should Know Time
Pfizer S Paxlovid Covid Pill Poised To Be Among Fastest Selling Treatments Ever Bloomberg

Paxlovid For A Patient On A Doac Ontario Covid 19 Science Advisory Table

Hoffnungstrager In Der Corona Therapie Paxlovid Neues Covid Medikament Rbb

Evaluating Differences In Viral And Symptom Rebounds Between Paxlovid Treated And Untreated Covid 19 Patients